Pipette tips are precision articles whose production is complex and technically demanding. In addition to a high-performance, repeatable injection molding machine, high-precision molds, fast robotics with valid part inspection, and automated networking of all components are required to manufacture them with the highest quality and cost-effectiveness. A new, fully coordinated turnkey system combines the specific requirements.
By Wolfgang Zangerle , Head of Business Development at Netstal
Due to the pandemic-related surge in demand, pharmaceutical and medtech manufacturers and suppliers are increasingly considering their own production of pipette tips. However, they often lack the technical background to build these themselves. Pipette tips for in-vitro diagnostics and other laboratory analysis must meet high quality standards and tight production tolerances. Specifically, the defined design tolerances must be met exactly in order to absorb the defined amount of liquid and transfer it without carryover.

In today’s automated liquid handling systems, capacitive liquid sensing is the predominant technology, which requires electrically conductive pipette tips. The perfect pipette tip (Fig. 1) is the result of an optimized production flow in which material, mold and production technology work together seamlessly.
Components of the production system
Complete production cells according to cGMP offer one option. The production centers can be continuously qualified and deliver both quality, production reliability and high volumes. Netstal demonstrated a reference system at K 2022: the all-electric machine of the ELION series has a clamping force of 120 tons in the medical version and works with an injection mold from Männer. The pipettes produced are gently and quickly removed from the mold by means of an automation system from MA micro automation and 100 percent optically inspected and dispensed in trays.
The production cell delivers fully tested “100 μl pipettes” from a 64-cavity mold in a very fast cycle time of 5.3 seconds. The production center requires only 25 m² of production area, which corresponds to a gross capacity of around 43,000 pipettes per hour, i.e. around 1720 pipettes per hour and square meter.
Requirements for the injection molding machine
The injection molding machine must fulfill specific tasks for pipette production. In material preparation, for example, an appropriate plasticization must be selected which does not contaminate the raw material, does not de-homogenize it or even degrades it impermissibly. If one of these factors is not controlled, the pipettes could fail in the automated diagnostic process, necessitate repetition or even lead to incorrect measurements.
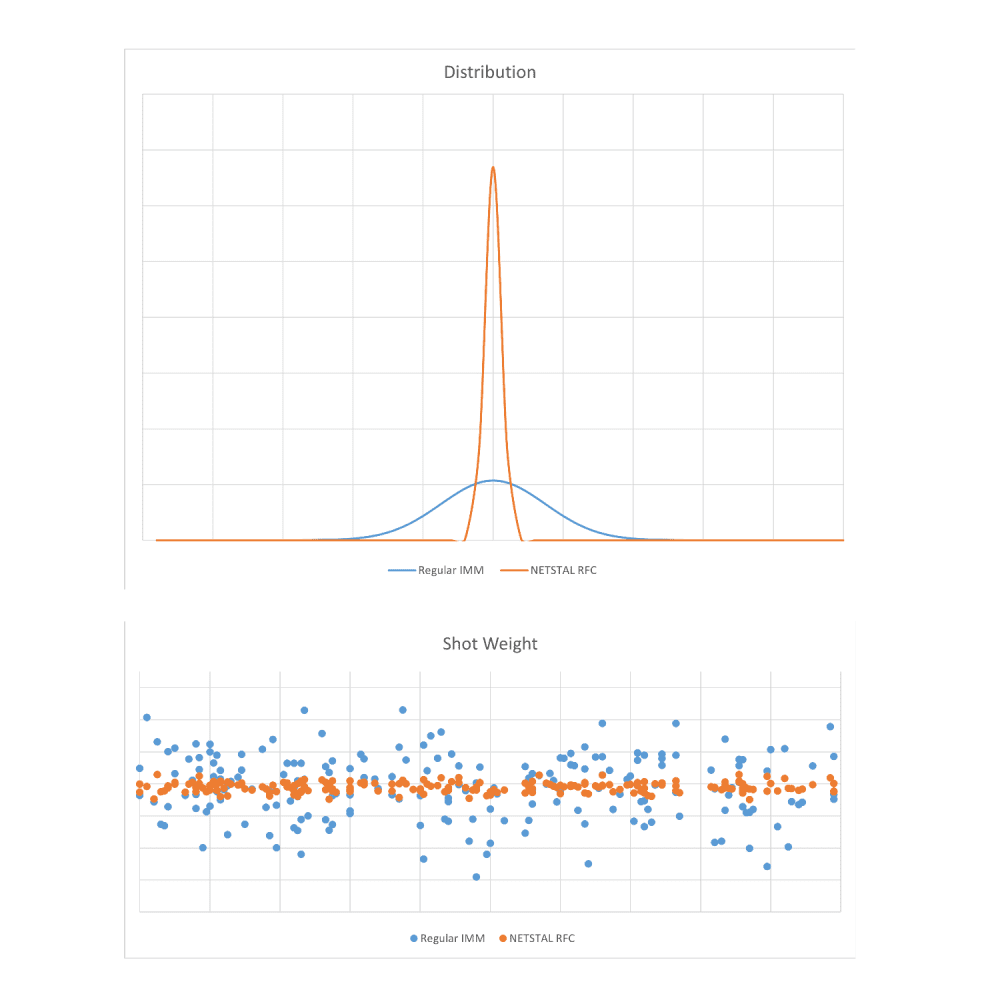
Netstal has devised a proprietary RFC control for accurate production results, which keeps the process of injection into the mold stable to produce high dimensional consistency at the pipettes. Figure 2 shows the difference in shot weight consistency for production with and without RFC control.
Precise mold
In addition to the high precision of Männer’s mold, the hot runner with its 64 nozzles must also be perfectly controlled and balanced in order to achieve symmetrical filling of the cavities and thus identical pipettes. This is ensured, among other things, by temperature sensors at all 64 hot runner nozzle tips, whose signals are passed on to the G24 Gammaflux controller. The controller records the values 20 per second and makes adjustments if they deviate 0.014°C from the setpoint.
HB-Therm temperature control units are used for high-precision temperature control of the entire mold and, in particular, of all 64 cavities. Supply temperature, pressure and flow are controlled. 16 flow sensors monitor the 64 cavities and the return heating. The result is identical caloric conditions for all cavities. Any deviation must be avoided, as dimensional changes on the pipettes lead to rejects during the subsequent optical inspection.
Connected via OPC UA
To ensure that the various components and systems act correctly in the network, they are networked by means of OPC UA interfaces. The operator of the production unit can control all system units via a central panel and be guided by SMART OPERATION, the automated start and stop program from Netstal. The mold is also an important element of production safety. Using predefined sequences, the control system can guide the operator through their operating operations. This is important when producing in a four-shift system.
As soon as the pipette tips have cooled down to removal temperature and the mold moves up, the modular automation system from MA micro automation with its high-speed linear axis goes into action. It removes the 64 pipettes within 1.07 seconds and transfers them on. Now a quality shot can be removed or the parts continue to the camera inspection.
Inspection and clean room
Visual inspection is just one of many inspections developed by MA micro automation for fully automated production lines for the manufacture of pipette tips. The 100% inspection monitors wobble/curvature of each pipette. 100% inspection of the pipette tip (frontal and radial) and the pipette holder (frontal) can be additionally applied in a controlled manner to make absolutely sure that no pipettes are placed in racks that do not exactly meet the defined part accuracy. During the inspection, up to 48 measurements/second are performed, and this in a cGMP qualified state. After successful inspection, the pipettes are filled into appropriate racks, which are injection molded or thermoformed, or into bags, depending on the customer’s desired configuration.
Pipette production is subject to the requirements of a controlled environment, mostly cleanroom ISO 8 and GMP C. A qualification of the turnkey plant according to cGMP and the fulfillment of the corresponding clean room requirements is therefore mandatory, here the calibration of the machine plays a decisive role. Netstal is certified according to ISO 17025 and is also allowed to perform recalibrations on production equipment at the customer’s site to ensure the production consistency and validity of the machine.
First turnkey production
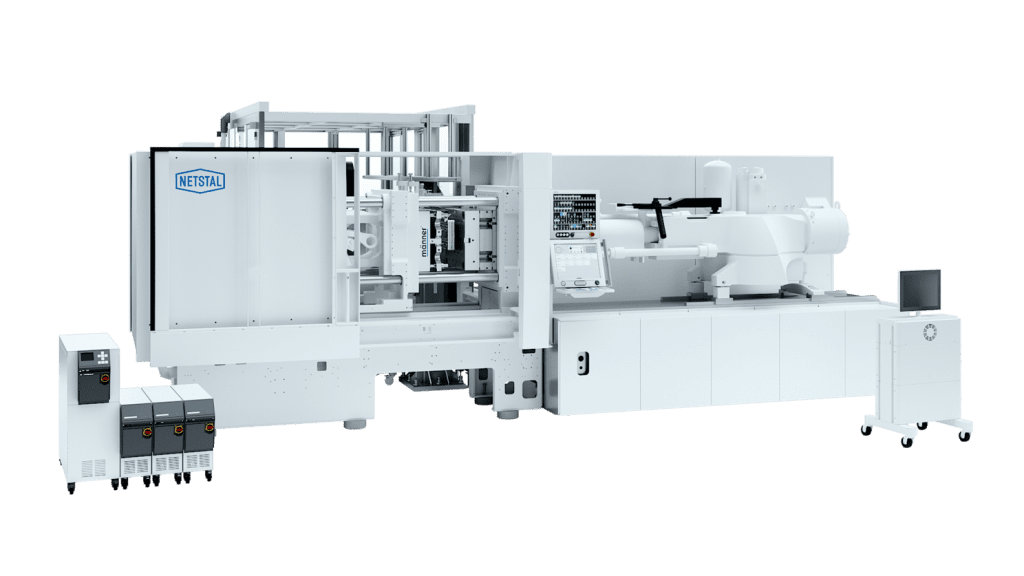
Until now, there has been no completely coordinated turnkey plant for pipette tips on the market. The novel production cell (Fig. 3) is an impressive result for hand-in-hand cooperation between the manufacturer and all suppliers involved. This makes it very easy for the customer to configure his own system. The time-consuming and cost-intensive trial-and-error phase is skipped; after successfully completed acceptance tests, the pipette cell is directly “ready to produce” as a system. In addition to a high level of safety when taking the step into the product segment, the new production island offers maximum efficiency and the highest output in series production.